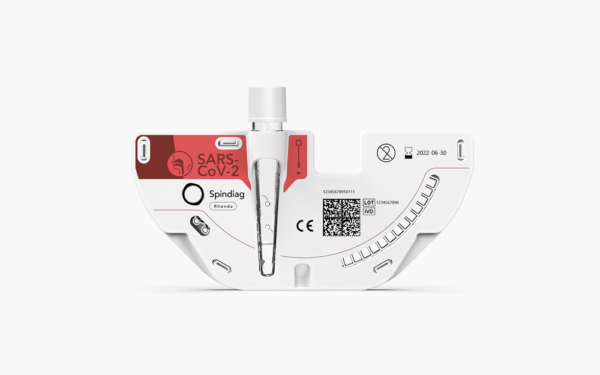
Rhonda SARS-CoV-2 disks are test cartridges for the Rhonda in-vitro diagnostic system and provide PCR test results with the Rhonda player fully automated in well under one hour.
The Rhonda SARS-CoV-2 test cartridges directly receive patient swab samples, no pipetting required.
The disks contain all reagents required to examine the inserted swab sample for the presence of the targeted pathogen, i.e. SARS-CoV-2 via E-Gene detection.
The disks feature an elaborate system of microchannels which facilitate the highly accurate sample processing and can be stored at room temperature.
OnePager – Direct Download
German Version
Ordering information Rhonda SARS-CoV-2 disk
Reference: SD003-02-020-A01
Packaging units = 1 box containing 20 disks
Mutations SARS-CoV-2
According to current knowledge, Rhonda also reliably detects SARS-CoV-2 with the following currently known mutations (based on published sequences in-silico).
The classification of mutations is differentiated between virus variants of concern (VOC) and variants under special observation (variants of interest, VOI), respectively variants under monitoring (VUM). Variants that differ relevantly in their properties (e.g. transmissibility or virulence) from conventional virus variants are defined as VOC.1,2
| Mutation1,2,3 | Definition4 | Rhonda disks for SARS-CoV-2 detection |
|---|---|---|
| B.1.1.7 (Alpha) | VOC | ✔ |
| B.1.351 (Beta) | VOC | ✔ |
| P.1 (Gamma) descending from B.1.1.28 | VOC | ✔ |
| B.1.617 (Delta) | VOC | ✔ |
| B.1.1.529 (Omicron) | VOC | ✔ |
| Omicron subvariants BA.1 (including BA.1.1) BA.2 BA.3 BA.4 BA.5 BQ.1.1 BF.7 XBB.1.5 | ✔ | |
| B.1.621 (My) | VOI | ✔ |
| C.37 (Lambda) | VOI | ✔ |
| BA.1 x AY.4 recombinant (XD) | VUM | ✔ |
| Mutation1,2 | Definition3 | Rhonda disks for SARS-CoV-2 detection |
|---|---|---|
| B.1.1.7 (Alpha) | VOC | ✔ |
| B.1.351 (Beta) | VOC | ✔ |
| P.1 (Gamma) descending from B.1.1.28 | VOC | ✔ |
| B.1.617 (Delta) | VOC | ✔ |
| B.1.1.529 (Omicron) | VOC | ✔ |
| B.1.1.529 BA.2 (Omicron sub variant) | ✔ | |
| B.1.621 (My) | VOI | ✔ |
| C.37 (Lambda) | VOI | ✔ |
[1] https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Virusvariante.html,last visited January,12th 2023
[2]weekly report https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Daten/VOC_VOI_Tabelle.html,last visited January,12th 2023
[3]https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Gesamt.html, last visited January,12th 2023
[1] https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Virusvariante.html, last visited January,12th 2023
[2] https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/, last visited January,12th 2023
[3] weekly report https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Daten/VOC_VOI_Tabelle.html, last visited January,12th 2023
[4] https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Gesamt.html, last visited January,12th 2023
Quality controls according to RiliBÄK 2019
Manufacturer: Microbiologics, Inc. | Distributor: Doenitz ProLab GmbH
Purchasing is exclusively done by the distributor Doenitz ProLab GmbH and is not part of the service of Spindiag GmbH.
Contact details of Doenitz ProLab GmbH:
Tel.: +49 821 440 159 0
Fax: +49 821 440 159 2
Business hours: 08:30 – 15:30 (Monday – Friday)
Email: info@doenitz-prolab.de
Homepage: www.doenitz-prolab.de
Positive control: Article No.: HE0066NS, Manufacturer: Microbiologics
Package Size: 1 Kit of 6 Swabs with Inactivated SARS-CoV-2 Whole Virus
Negative control: Item No.: HE0067NS, Manufacturer: Microbiologics
Package Size: 1 Kit of 6 Inactivated Swabs Negative Cellularity Control
From Swab to Result in well under one hour
The Rhonda SARS-CoV-2 disks are part of the Rhonda in-vitro diagnostic system. Run the analysis fully automated in combination with the Rhonda player.
